Abstract
Background
Venous thromboembolism (VTE) is a major cause of morbidity and mortality in patients with cancer. Despite therapeutic anticoagulation, the risks of recurrent VTE and major bleeding are approximately 10% and 5%, respectively, during the first 6 months of treatment. Overall mortality ranges from 25% to 40%, depending on the study population. Knowing the case fatality rates of these outcomes is also important for weighing the relative risks and benefits of anticoagulation in patients with cancer-associated VTE but these rates have not been reported previously.
Objective
To determine the incidence of recurrent VTE and major bleeding events and to calculate the case fatality rates of these outcomes in patients undergoing anticoagulation for cancer-associated VTE.
Methods
An electronic search of MEDLINE, EMBASE and the Cochrane Central Register of Controlled Trials from January 1980 to May 2018 was performed. English language publications (observational studies and randomized controlled trials [RCTs]) that reported on patients with active cancer and VTE who received anticoagulation with low molecular weight heparin (LMWH), vitamin K antagonist (VKA), or a direct oral anticoagulant (DOAC) for at least 3 months were retrieved for review. In addition, a hand search of references of review articles was done to complement the electronic literature search. Studies that provided information on recurrent VTE, major bleeding events, mortality, and causes of death were included in analyses. Retrospective studies and prospective cohorts with fewer than 50 patients were excluded. Two reviewers independently screened for study eligibility and extracted data onto standardized forms. Study outcomes were recurrent VTE, major bleeding and death. Pooled proportions with 95% confidence intervals (CI) were calculated according to anticoagulant treatment and study design.
Results
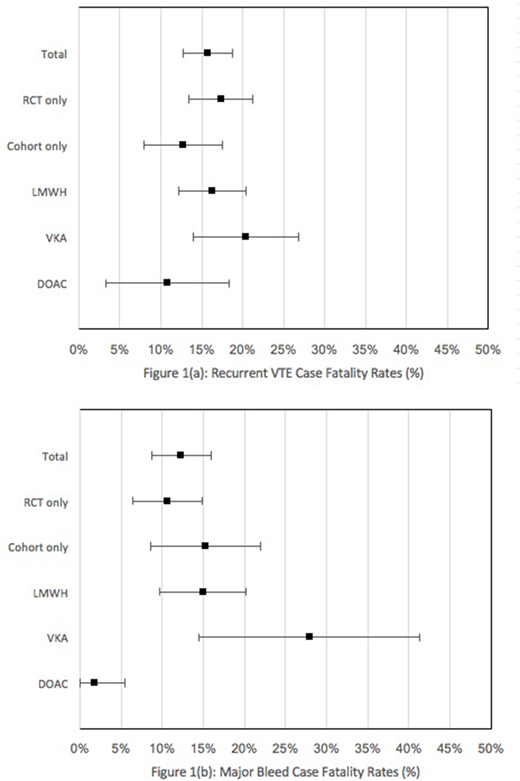
The search identified 7327 studies of which 29 studies (15 prospective cohort studies and 14 randomized controlled trials) were included. Data from 8000 cancer patients followed for a total of 4786 patient-years (range 3 to 36 months) were summarized. The rate of recurrent VTE and fatal recurrent VTE were 15.7% (95% CI, 14.4% to 17.1%) and 2.5% (95% CI, 2.0% to 3.0%) per patient-year of follow-up, respectively, with a case fatality rate of 15.8% (95% CI, 12.7% to 18.8%). A sub-analysis revealed case fatality rates for recurrent VTE to be 16.3% (95% CI, 12.2% to 20.4%) for LMWH, 20.4% (95% CI, 14.0% to 26.8%) for VKA, and 10.8% (95% CI, 3.2% to 18.3%) for DOAC therapies. The rate of major bleeding and fatal major bleeding events were 6.4% (95% CI, 5.5% to 7.3%) and 1.2% (95% CI, 0.8% to 1.6%) per patient-year of follow-up, respectively, with a case fatality rate of 12.3% (95% CI, 8.7% to 15.9%). A sub-analysis revealed case fatality rates for major bleeding events to be 14.9% (95% CI, 9.6% to 20.2%), 27.9% (95% CI, 14.5% to 41.3%), and 1.9% (95% CI, 0% to 5.5%) for LMWH, VKA, and DOAC therapies, respectively. Among RCTs, case fatality for recurrent VTE was 17.3% (95% CI, 13.5% to 21.2%) and for major bleeding was 10.8% (95% CI, 3.2% to 18.3%). Among prospective cohort studies, respective case fatality rates were 12.8% (95% CI, 8.0% to 17.5%) and 15.3% (95% CI, 8.6% to 22.0%). Studies were heterogeneous in the duration of follow up and their reporting of the causes of death and definition of fatal PE.
Conclusion
The incidences of recurrent VTE and major bleed events are high in patients with cancer-associated VTE on anticoagulant therapy. Case fatality from recurrent thrombosis is higher than the case fatality from major bleeding. Differences among various anticoagulants likely reflect patient selection bias and heterogeneity of studies.
Lee:BMS: Research Funding; Bayer: Consultancy, Honoraria; LEO Pharma: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Servier: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal